Which of the Following Structures Contains a Secondary Amine
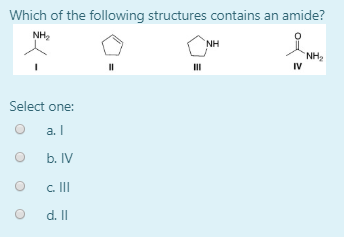
Which of the following structures contains an amide. Has two and a tertiary 3 amine A.

What Are The Reactions For Amines And Amides Example
Amines contain nitrogen and are commonly found in a wide variety of.
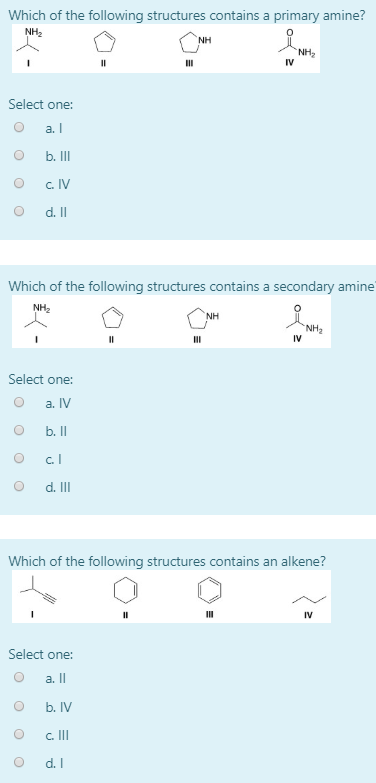
. Ammonia primary secondary tertiary amine amine amine. Aliphatic amines contain only H and alkyl substituentsAromatic amines have the nitrogen atom connected to an aromatic ring. NH2 IV III Question 71 Which of the following compounds has the lowest boiling point.
- So the correct option is A. C N - CH - CH3 here N is attach three carbon. They are bound to the nitrogen together with one hydrogen.
Acyclic alkanes have two fewer H atoms than cyclic alkanes with the same number of carbons. A common example includes dimethylamine. Thus option B is the correct answer.
The reaction below is an example of an acid-base reaction. It also tertiary amine C. Amines can be classified according to the nature and number of substituents on nitrogen.
So it is tertiary amine B. - The amine group is involved in the cyclic ring. Numerous heterocyclic amines are found in biochemical systems heterocyclic amine are amine in which nitrogen is one of the atoms of a ring.
A I B II C III D IV. Secondary Tertiary amines are generally more potent in blocking reuptake of serotonin while secondary amines are more potent in blocking the reuptake of norepinephrine. Structure of trimethyl amine CH3 H2 C - N - CH3 here.
So it is secondary amine D. Based on the number of hydrogen atoms replaced amines are classified into three. Which ones have each of these functional groups in there are groups.
The nitrogen atom has a methyl group and a propyl group so the compound is methylpropylamine a secondary amine. Likewise diphenylamine is an example of an aromatic amine. NH 수 om III IV.
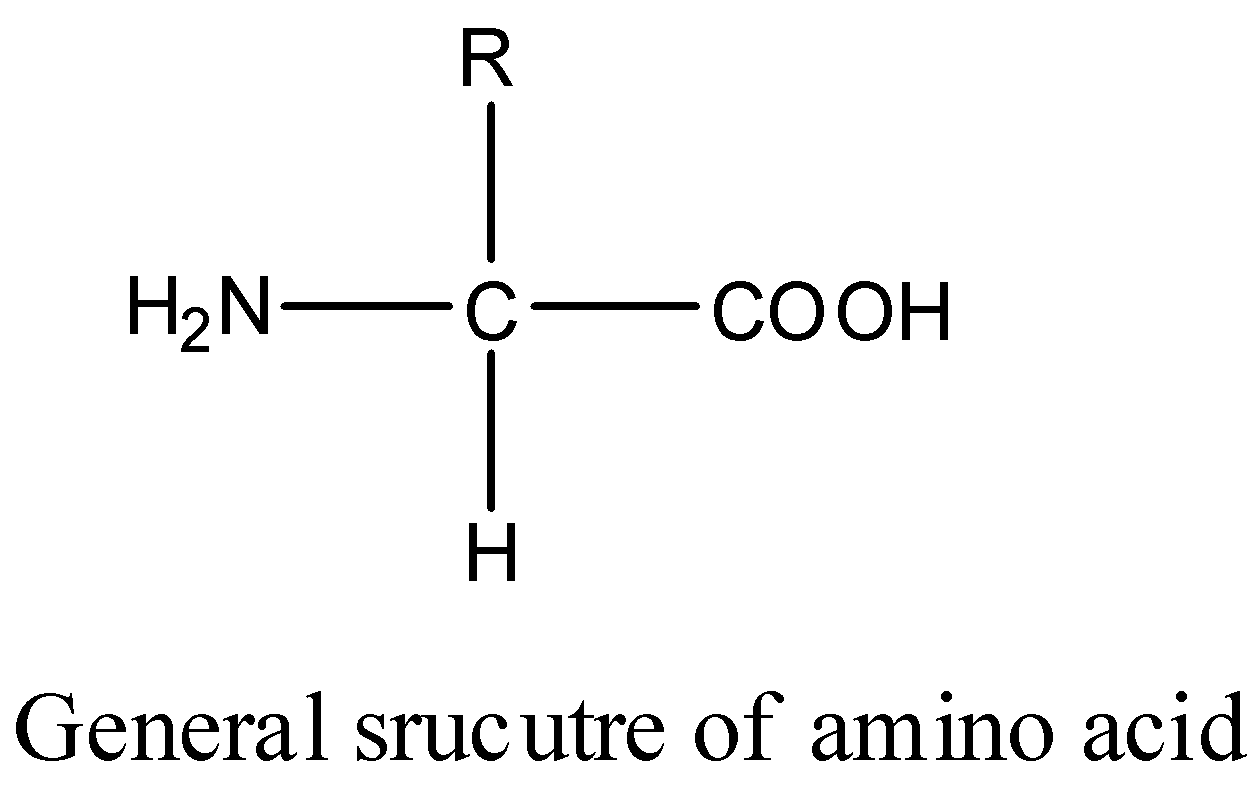
So remember that the structure of amino acid is a central carbon with a hydrogen a car box like acid amedo group and then in our group. Rank the following compounds in order of increasing strength of intermolecular forces putting the molecule with the weakest intermolecular force first. The following formula is used to represent tertiary amines.
DEA is a secondary amine and is also used to treat natural gas to pipeline specifications. Nortriptyline is a metabolite of amitriptyline protriptyline and desipramine is a metabolite of imipramine. 9-11 98 Naming Amines Common names are often used to name simple amines.
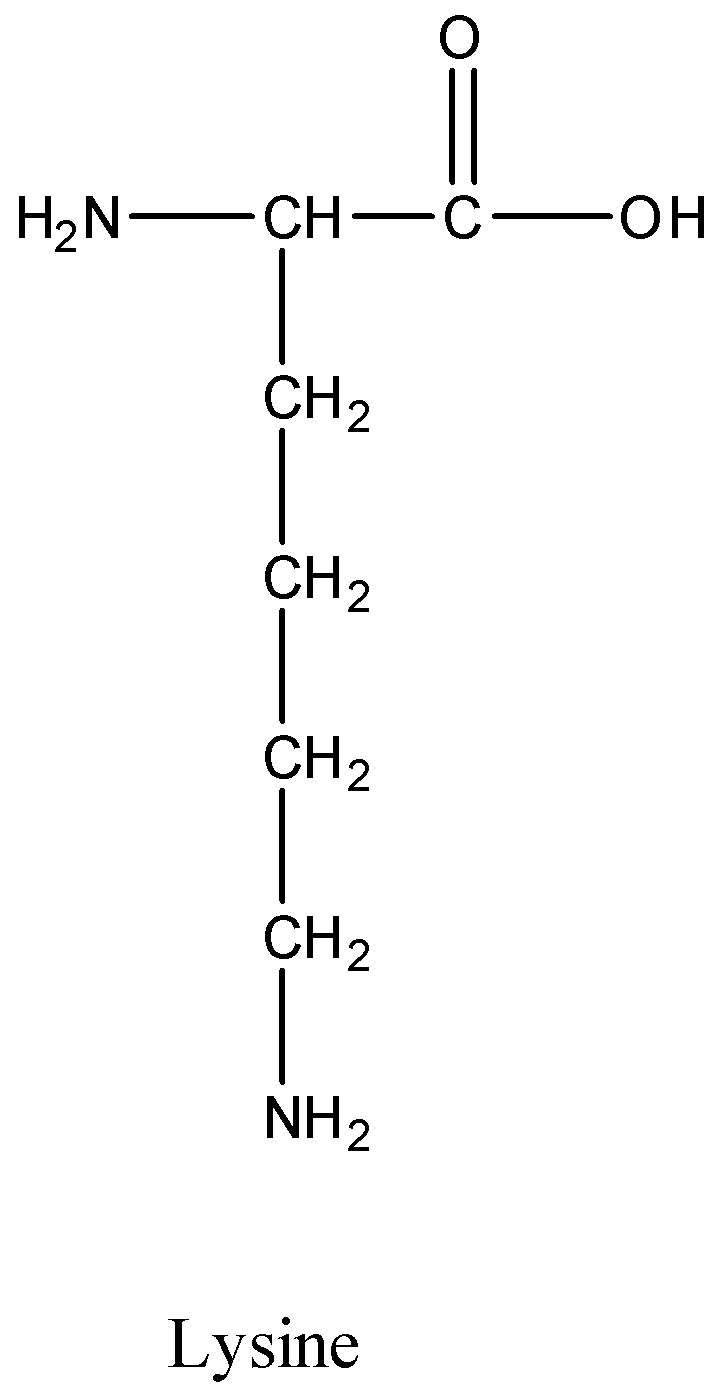
NH2 NH NH2 II II IV А I B II С I D IV 14. C H 3 N H C H 3 represents R N H R. Lysine is one of the amino acids containing two primary amine groups in its structure and it is an essential amino acid.
Alkanes are aliphatic hydrocarbons. The compound is ethyldimethylamine a tertiary amine. Structure of NIN-dimethylethylamine CH3 H.
The amine is secondary so the compound is diethylamine. Alkanes contain only C-C and C-H sigma bonds. Primary Secondary and Tertiary amines.
A I B II C III D IV. The nitrogen atom has a methyl group and a propyl group so the compound is methylpropylamine a secondary amine. - Therefore Proline is the amino acid containing secondary amine in its structure.
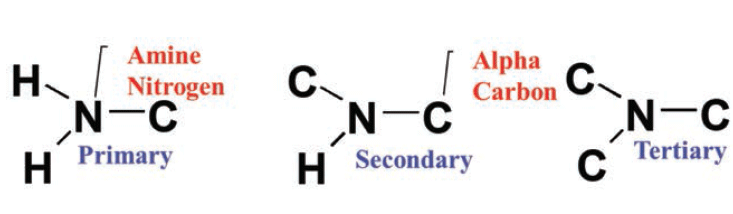
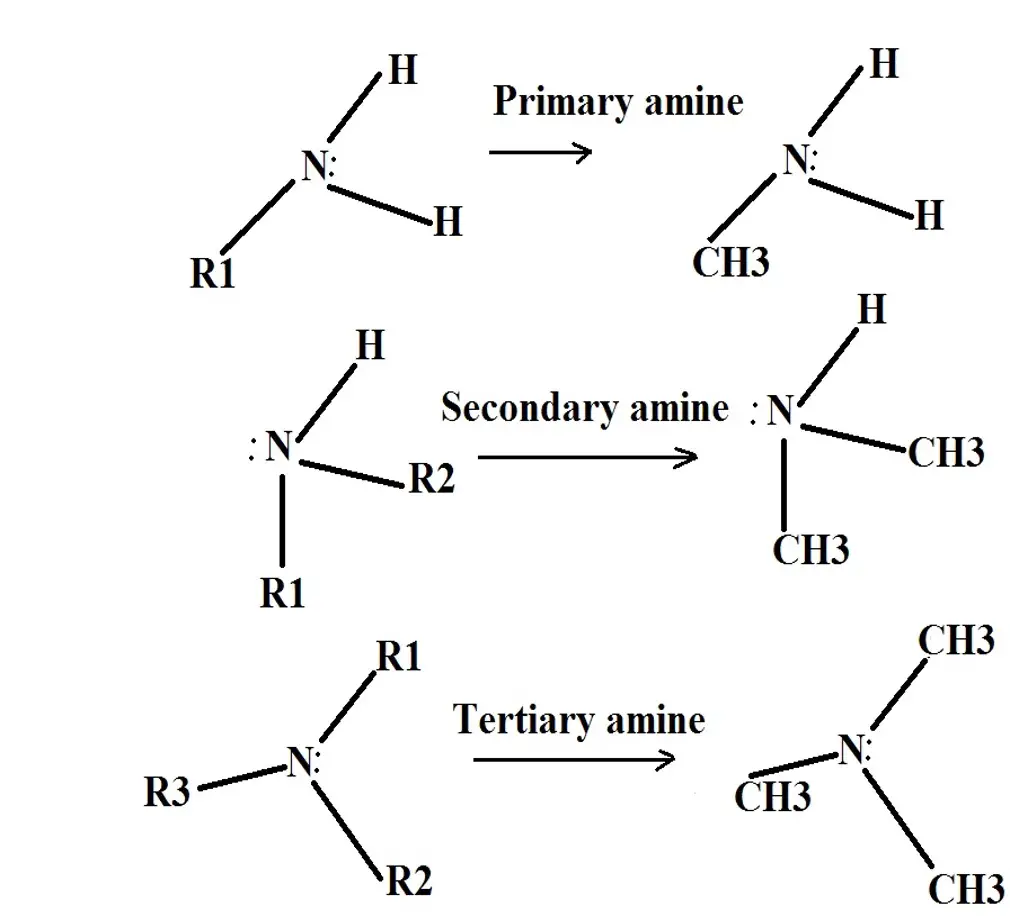
Which of the following structures contains a primary amine. Alkanes are acyclic or cyclic. A primary 1 amine A compound that has only one alkyl or aryl group on the nitrogen atom.
Structure of 2-Chloro- 3- methyl- 2 heptylamine 7 6 3 CJ He - C12 - CH - thy - CH - C -. Structure and Bonding 20. Examples Methylamine a 1 amine Trimethylamine a 3 amine CH 3 CH 3 CH 3-NH 2 CH 3-N.
In secondary amine 2 alkyl groups and one hydrogen atom are directly attached to N atom. Secondary amines are formed when two hydrogen atoms of ammonia or one hydrogen atom of R-NH 2 are replaced by an alkyl or aryl group. ИФИОН 111 IV Question 72 Which of the following compounds is expected to be the most soluble in H20.
Primary left 1rmo right Amines. There are two methyl groups and one ethyl group on the nitrogen atom. Cyclic amines are either secondary or tertiary amines which are designated as heterocyclic compounds.
This question asked us to look at the table of amino acids provided by the book and German. Group C is a secondary amine where the hydrogen can act as a hydrogen bond donor and the nitrogen can act as a hydrogen bond acceptor. A compound with molecular formula C8H10BrN exhibits a singlet at 12 2H a triplet at 28 2H a triplet at 30 2H a doublet at 71 2H and a doublet at 74 2H in its 1H NMR spectrum.
So this is what were looking at is the our group to figure out which ones have. Which of the following structures contains a secondary amine. I II III IV Which of the following statements about alkanes is not true.
There are two ethyl groups attached to the nitrogen atom. DEA systems do suffer the same corrosion problems but not as severely as those using MEA. Which of the following structures contains a secondary amine.
There are two ethyl groups attached to the nitrogen atom. Which of the following structures contains a secondary amine. Which of the following structures contains a secondary amine.
Amines alkyl and aryl alike are organized into three subcategories see table based on the number of carbon atoms adjacent to the. NH2 NH NH2 IV A I B II С Ш D IV. Which of the following structures contains a primary amine.
Structure of diethylamine Hy C- th - N - CH - CH3 here IN is attach two carbon. Categorize each of the following amines as either a primary amine secondary amine or tertiary amine. This is because all the hydrogen atoms of the ammonia molecules are replaced by R groups.
Solution strengths are typically from 25 to 35 DEA by weight in water. Science Chemistry QA Library 13. Unlike primary and secondary amines tertiary amines do not have any hydrogen atoms.
Group D is an alcohol where the hydrogen can act as a hydrogen bond donor and the oxygen as a hydrogen bond acceptor. It is represented by general formula R 2 N H. Tertiary left 3rmo right Amines.
It contains only one alkyl or aryl group attached to the nitrogen atom. Because of the presence of an amino group in the cyclic structure proline is called imino acid. A I B II C III D IV.
As a secondary amine DEA is less alkaline than MEA. Has one alkyl or aryl group on the nitrogen atom a secondary 2 amine A compound that has two alkyl or aryl groups on the nitrogen atom. Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom.
Provide the reagents necessary to carry out the following conversion. R3N where the nitrogen contains a free pair of electrons. The amine is secondary so the compound is diethylamine.
Secondary amine TCAs include. If two or three alkyl groups are. Secondary left 2rmo right Amines.
Predict the major product for the following reaction. Tertiary Amines 3 0 Amines. Secondary Amines 2 0 Amines Secondary amines are those amines that have two organic substituents either alkyl or aryl ones or both.
Primary amines are formed when one hydrogen atom of ammonia is replaced by R or Ar. 95 Assign names to each of the following amines.
Organic Nitrogen Compounds Iii Secondary And Tertiary Amines
Which Amino Acid Contains Secondary Amino Groups In Class 12 Chemistry Cbse
How To Identify And Classify Amines Examples And Characteristics

Organic Nitrogen Compounds Iii Secondary And Tertiary Amines
Solved Which Of The Following Structures Contains A Primary Chegg Com

1 Draw And Name A Primary Secondary And Tertiary Amine And A Primary Secondary And Tertiary Amide Draw No Name Necessary A Quaternary Ammonium Cation All Molecules S Study Com
Which Amino Acid Contains Secondary Amino Groups In Class 12 Chemistry Cbse
Identification Of Amines Identification Of Primary Secondary And Tertiary Amines By Hinsberg Test Hoffman Mustard Oil Reaction

Amine Chemical Compound Britannica

Which Of The Following Is A Secondary Amine
Solved Which Of The Following Structures Contains A Primary Chegg Com
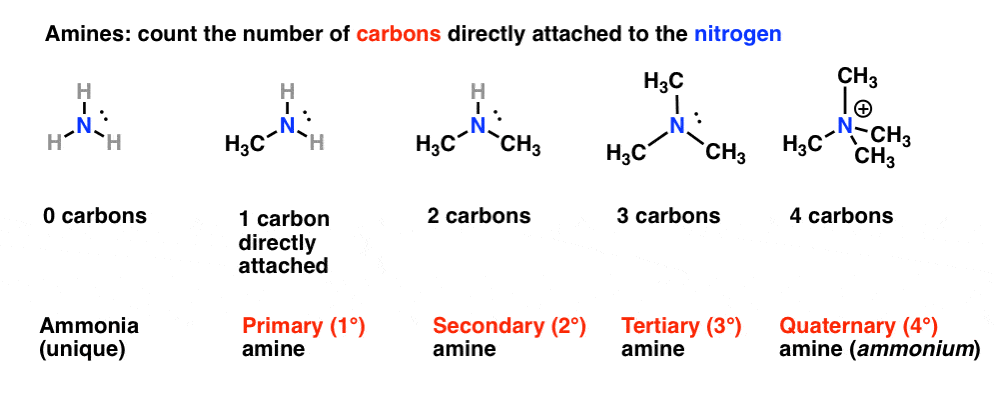
Primary Secondary Tertiary And Quaternary In Organic Chemistry
Chapter 5 Amines And Amides Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library
Illustrated Glossary Of Organic Chemistry Secondary Amine
Difference Between Primary Secondary And Tertiary Amines Definition Basicity Examples Differences

Amine Definition Structure Reactions Formula Video Lesson Transcript Study Com
Chapter 5 Amines And Amides Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library
